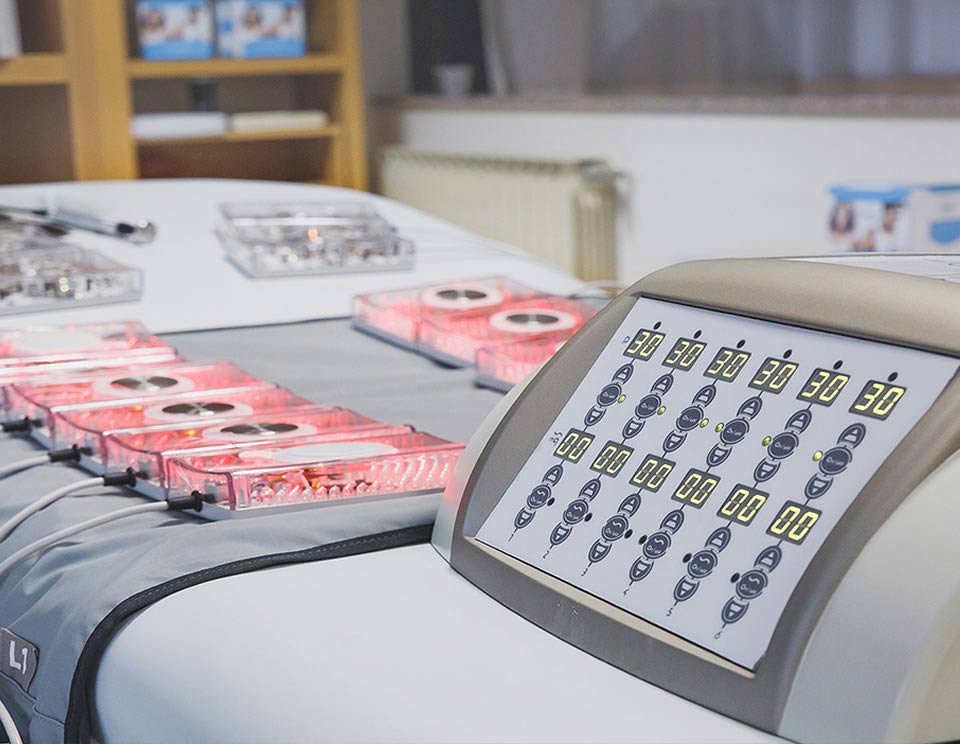
Aesthetic and medical sector
Machinery and semi-finished products
The technological evolution acquired in industrial automation allows us to design, develop and produce highly innovative and competitive customised solutions to customer specifications.
Our processes are compliant with ISO 13485 and our products comply with statutory requirements (EU Regulation 2017/745 - Medical Devices, EEC Directive 93/42, IEC 62304, EN 60601, etc...).
The aesthetic and medical business unit at Electra deals mainly with OEM production, customised for each individual customer; it also supplies a line of ready-to-market electro-medical devices.
Our processes are compliant with ISO 13485 and our products comply with statutory requirements (EU Regulation 2017/745 - Medical Devices, EEC Directive 93/42, IEC 62304, EN 60601, etc...).
The aesthetic and medical business unit at Electra deals mainly with OEM production, customised for each individual customer; it also supplies a line of ready-to-market electro-medical devices.
Beauty
- Diode laser for hair removal - EPILCRIO 808
- Bipolar resistive radiofrequency with electroporation - SHINEFACE
- Vacuum bipolar resistive radiofrequency with LED photo-stimulation - SHAPEBODY
- Pressure massage - LINFOMASSAGE
Access to the viewing of the contents of the website www.electra.sm in this section is reserved for operators in the sector in compliance with current legislation (Decreto Legislativo 24 febbraio 1997, n.46).